Articles

This report was prepared for: PlasmaShield
This report was prepared by:
Dr Harriet Whiley
Associate Professor in Environmental Health
Flinders University
Level 5 Health Sciences Building, Bedford Park, Flinders University, 5042, SA
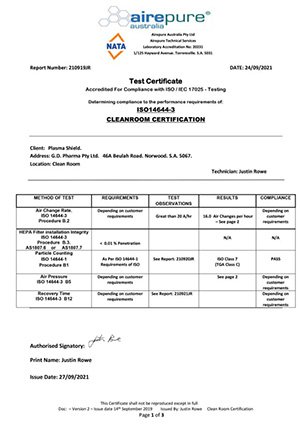
Healthy Buildings International Pty Ltd (HBI) was commissioned by UL Verification Services Inc. to perform an Indoor Air Quality Assessment of the Better Medical Centre located at 15 Victoria Cres, Mount Barker SA during the month of March 2023.
Based on the observations and results of the assessment HBI considers that indoor air quality in the building was acceptable as it met Australian Standards, with common indoor air pollutants either not detected or present at acceptably low concentrations.
The air handling units were found to be in good condition and well maintained. Inspection and assessment of the air filters and air supply ducts found them to be in good condition.
Tests conducted to assess various building air flow patterns showed that the building was positively pressurised and internal air flows were satisfactory. No visible stains or discolouration consistent with microbial growth were noted and no excessive dampness was identified using the thermal imaging camera.
Sampling and analysis of the building air for formaldehyde gas returned results that were below both the World Health Organization’s recommended guideline of 0.08ppm and the HBI adopted criterion of 0.3 ppm. They are therefore considered to be satisfactory.


Advanced air treatment systems have the potential to reduce airborne infection risk, improve indoor air quality (IAQ) and reduce energy consumption, but few studies reported practical implementation and performance. PlasmaShield® , an advanced multi-modal HVAC-integrated system, was directly compared with a standard MERV-13 system in a post-surgical paediatric healthcare setting. The evaluation entailed monitoring of multi-size airborne particles, bioaerosols and key IAQ parameters. Measurements were taken for outside air, supply air and air in the occupied space for 3 days prior to, and after, the installation of the PlasmaShield system. Compared with the existing arrangement, very significant reductions in particle number concentrations were observed in the occupied space, especially with virus-like submicron particles. Significant reductions in airborne culturable bacteria and fungi were observed in the supply air, with more modest reductions in the occupied space. In the case of virus-like particles, there was an eight-fold improvement in equivalent clean air, suggesting a five-fold infection risk reduction for long-range exposure. The data suggest multiple benefits of airborne particle and bioaerosol reduction, with applications beyond healthcare. Long-term studies are recommended to confirm the combined IAQ, health and energy benefits.
Airborne microorganisms play a significant role in the transmission of infectious diseases. As such, improving indoor microbial air quality can enhance infection control in numerous settings. This study examined the efficacy of the PlasmaShield® air purification device to remove airborne microorganisms under laboratory conditions. Pure cultures of model microorganisms at varying concentrations were aerosolized using a 1-jet Collison nebulizer through stainless-steel removable piping prior to reaching the PlasmaShield® device. The surviving microorganisms were captured using the Staplex® MBS-6 Six Stage Microbial Air Sampler and enumerated via culture on agar plates. The positive-hole-corrected colony/plaque-forming units were compared with the negative control (microorganisms aerosolized through an empty PlasmaShield® casing). The PlasmaShield® statistically significantly (p < 0.05) reduced airborne Escherichia coli, Staphylococcus epidermidis, Bacteriophage MS2 and Cladosporium sp. compared with the negative control. The maximum removal achieved was estimated to be 4 × log10 E. coli (99.99% removal), 4 × log10 S. epidermidis (99.97% removal), 7 × log10 MS2 (99.99998% removal) and 5 × log10 Cladosporium sp. (99.999% removal). Scanning electron microscope images of the surviving microorganisms showed that the PlasmaShield® damaged the cell membrane of these model microorganisms. This study provides proof-of-concept evidence to support the use of this technology to improve indoor microbial air quality.


The efficacy of the PlasmaShield device to inactivate aerosolised HCoV229E virus was tested. Purified HCoV229E, at a concentration of 1.93 x 108 TCID50/mL in PBS, was nebulised for 3 min through 21.5 cm of piping prior to the PlasmaShield and 21.5 cm piping after the PlasmaShield. For the baseline/negative control measurement, the PlasmaShield was replaced with an empty case. Surviving virus aerosols were collected in a liquid impinger sampler. The virus concentration was then determined by infectivity of Huh-7 cells and the TCID50/mL calculated. All experiments were conducted in triplicate. The baseline experiment (empty PlasmaShield case) detected 7.85 x 106 TCID50/mL HCoV229E. This was reduced by approximate a 1.5 x log10 (95% reduction) to 3.9 x 105 TCID50/mL HCoV229E with the PlasmaShield turned on.
Experiments were conducted to measure the particle collection and removal efficiencies of a non-thermal plasma air filtration system (PFS). This system utilises a uniform and high intensity electric field to produce low-medium energy electrons, which in turn can charge particulate matter (PM) that passes through the field, potentially causing them to agglomerate and be more readily captured by conventional porous media filters. The experiments comprised of single-pass efficiency measurements and smoke recovery tests, where in both cases the particle count across the size range of 0.3 µm to 10 µm was measured. The results show that when the PFS is paired with a conventional MERV13 post-filter, the particle collection efficiencies exceeded 99.95% for all measured particle sizes for filter face velocities < 1 m/s. When the post-filter was removed, the minimum efficiencies reduced to 76.98%. The results also show that the PFS set
at a flow-rate of 30 ach removed 99.8% of the smoke introduced into the room within 15 minutes.


Final Technical Report
In a previous Interim Report (AESHDP0122), Adelaide Exposure Science and Health (University of Adelaide) reviewed selected experimental data pertaining to the PlasmaShield Air Purification System (PAPS) and provided commentary on air purification from an occupational hygiene perspective. This report presents findings from an independent empirica

Potential of the Plasma Shield System in Reducing Energy and in Contributing Towards Green Buildings
A desktop analysis on the potential of Plasma Shield’s air purification system (PSS) to allow for a reduction in outside (“fresh”) air requirements based on Australian Standards 1668.2 – 2012, and the resultant energy savings that could potentially be realised, has been conducted under simplified conditions relevant to commercial office buildings. This analysis also includes a discussion on the amount of points that could potentially be awarded through the use of the PSS under different rating schemes, namely Green Building Council of Australia’s Green Star scheme and the international WELL Building Standard.
This case study showcases how PlasmaShield® technology was implemented in a large timber yard facility to significantly reduce HVAC peak load, energy consumption, and operational costs while maintaining superior indoor air quality. The results demonstrate the effectiveness and efficiency of PlasmaShield® in delivering sustainable, performance-based ventilation design without compromising comfort or compliance.


Learn how we implemented cutting-edge air purification technology to reduce airborne health risks and safeguard patient well-being, building on proven success from leading medical centers.
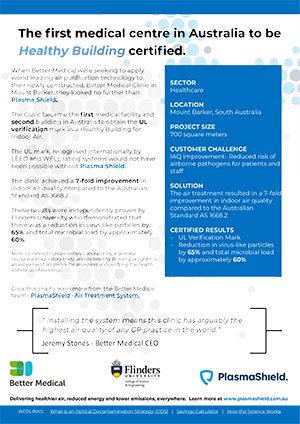
Learn how Better Medical partnered with us to integrate advanced health-focused solutions, leading to their status as Australia’s first Healthy Building certified medical centre. Explore the tangible improvements in facility quality and patient care detailed in this study.

Is your Building Management System (BMS) an unsung hero? Discover how to unlock its hidden potential to drive significant energy savings, reduce carbon footprint, and transform your property into a peak-performing, sustainable asset.
Address:
Se 15, Bulleen Corporate Center, Bulleen, VIC 3105
Email:
Phone:
+61 (0) 3 9404 5141